The modern definition of enzymology is synonymous with the Michaelis-Menten equation instituted by Leonor Michaelis and Maud Menten. Most textbooks, or chapters within, discussing enzymology start with the derivation of the equation under the assumption of rapid equilibrium (as done by Michaelis-Menten) or steady state (as modified by Briggs and Haldane) conditions to highlight the.. The more scientists understand the workings of enzymes, the better they understand the complex array of reactions that make up the web of life, in healthy and in diseased states. Seminal work published in 1912 by Leonor Michaelis (1875-1949) and Maud Leonora Menten (1879-1960), a German man and a Canadian woman, cast light on the reasons.

4.2a Derivation of the MichaelisMenten Equation Chad's Prep®
Cinétique enzymatique Equation Michaelis Menten YouTube

AS Biology The MichaelisMenten Constant (Km) YouTube
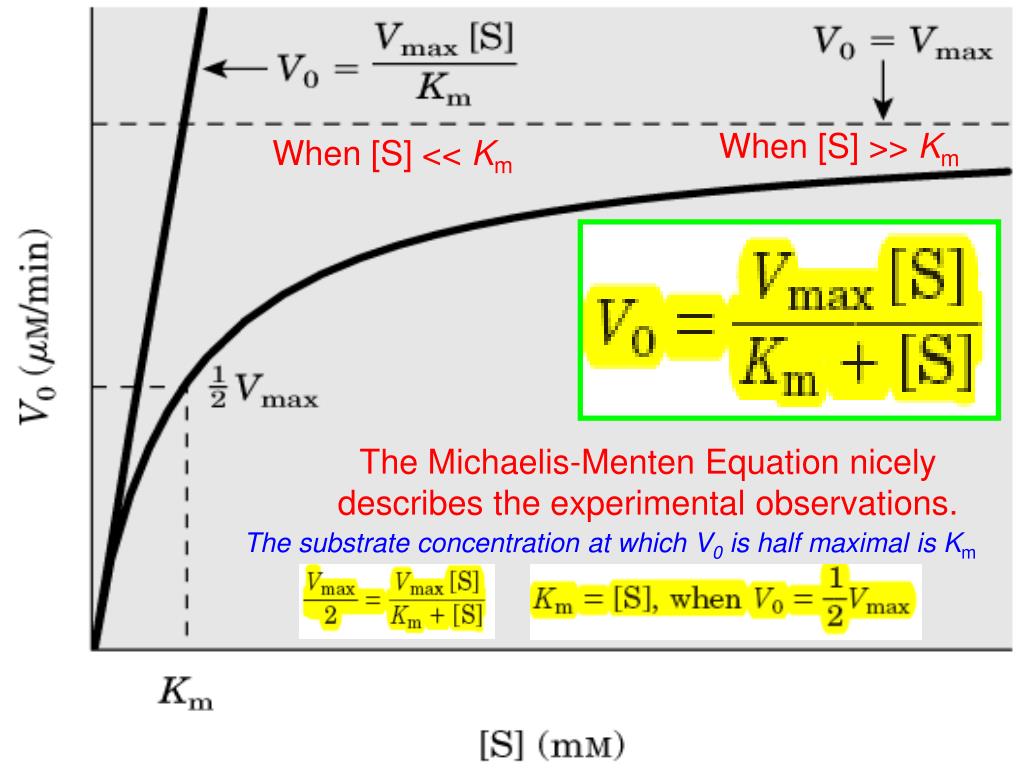
PPT The MichaelisMenten Equation nicely describes the experimental observations. PowerPoint
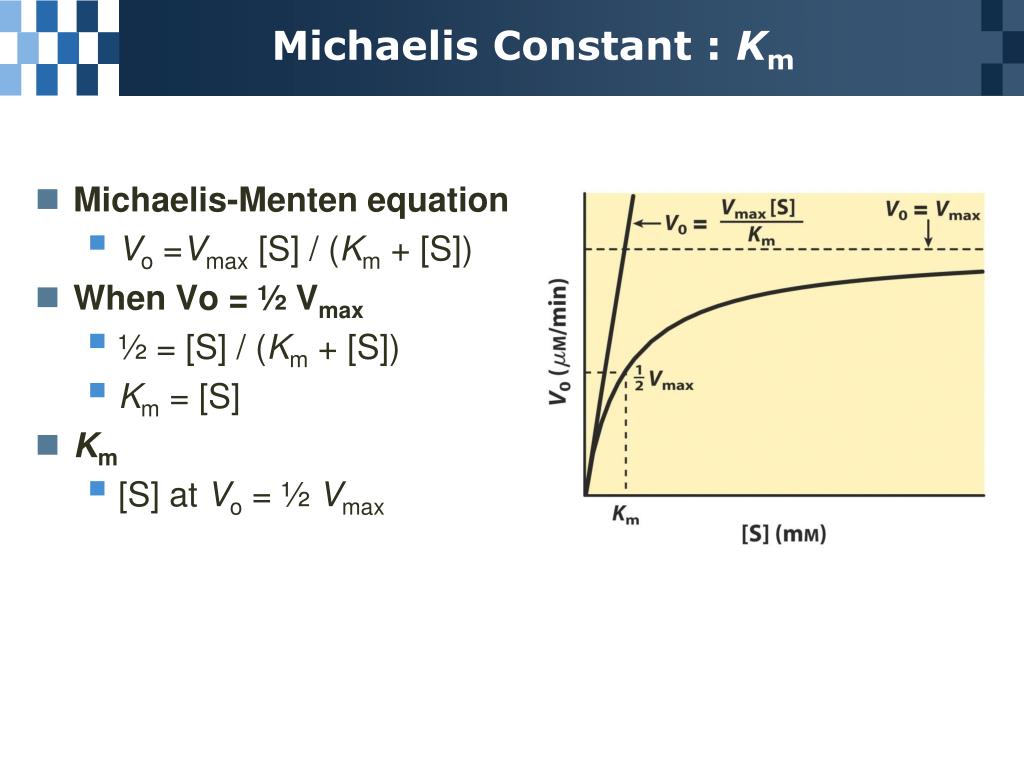
PPT Chapter 6 Enzyme PowerPoint Presentation, free download ID5692414
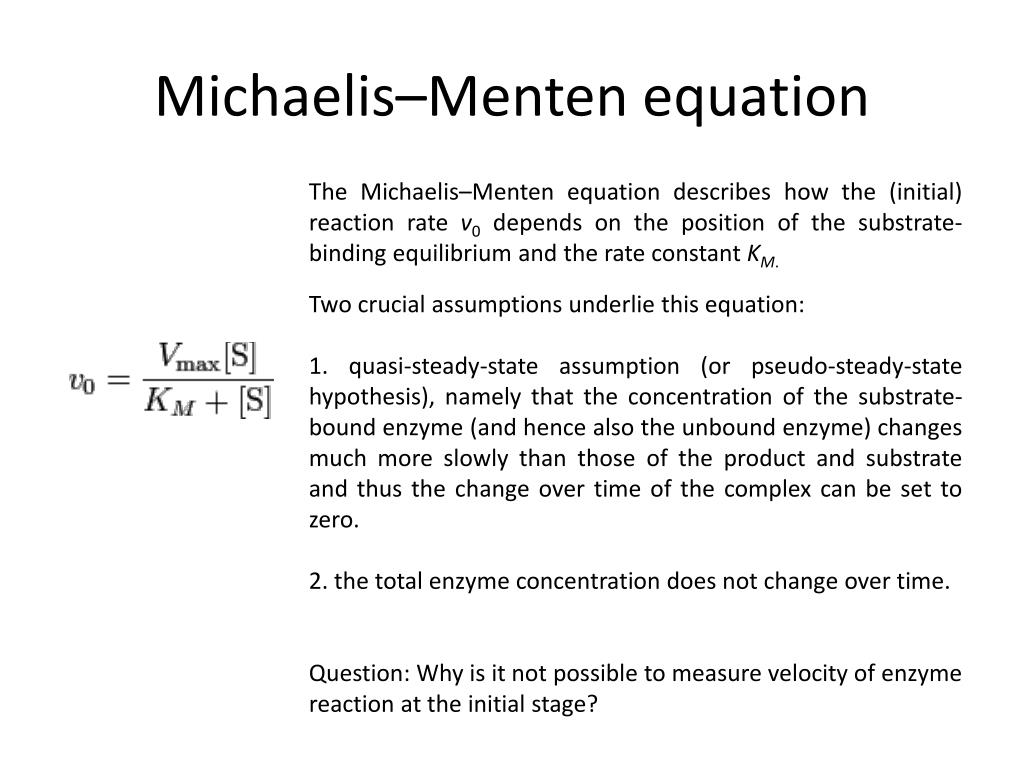
PPT Lecture 4 PowerPoint Presentation, free download ID2832807

Equation de Michaelis et Menten YouTube

MichaelisMenten equation in easy way YouTube
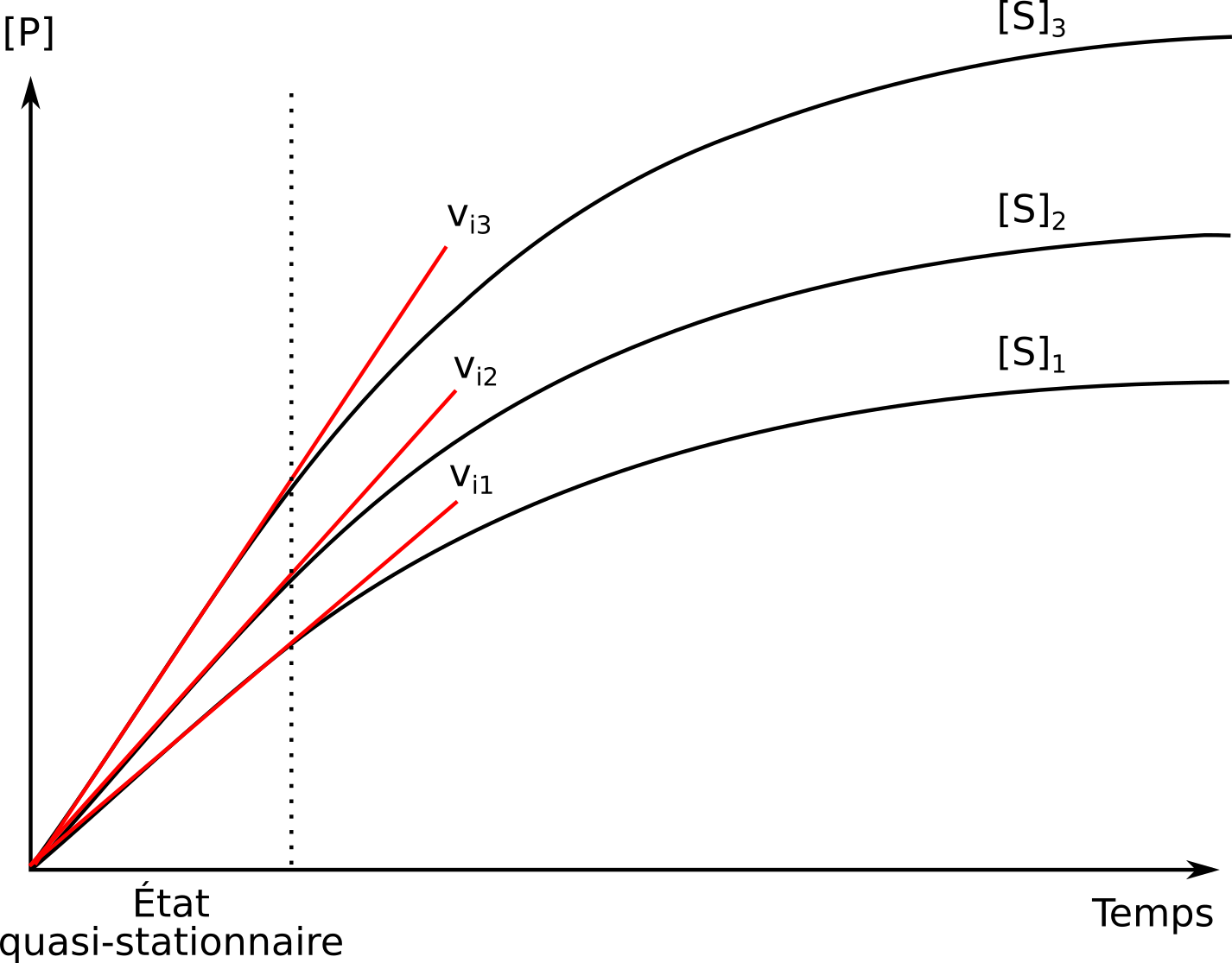
La cinétique des enzymes michaeliennes et l'équation de MichaelisMenten
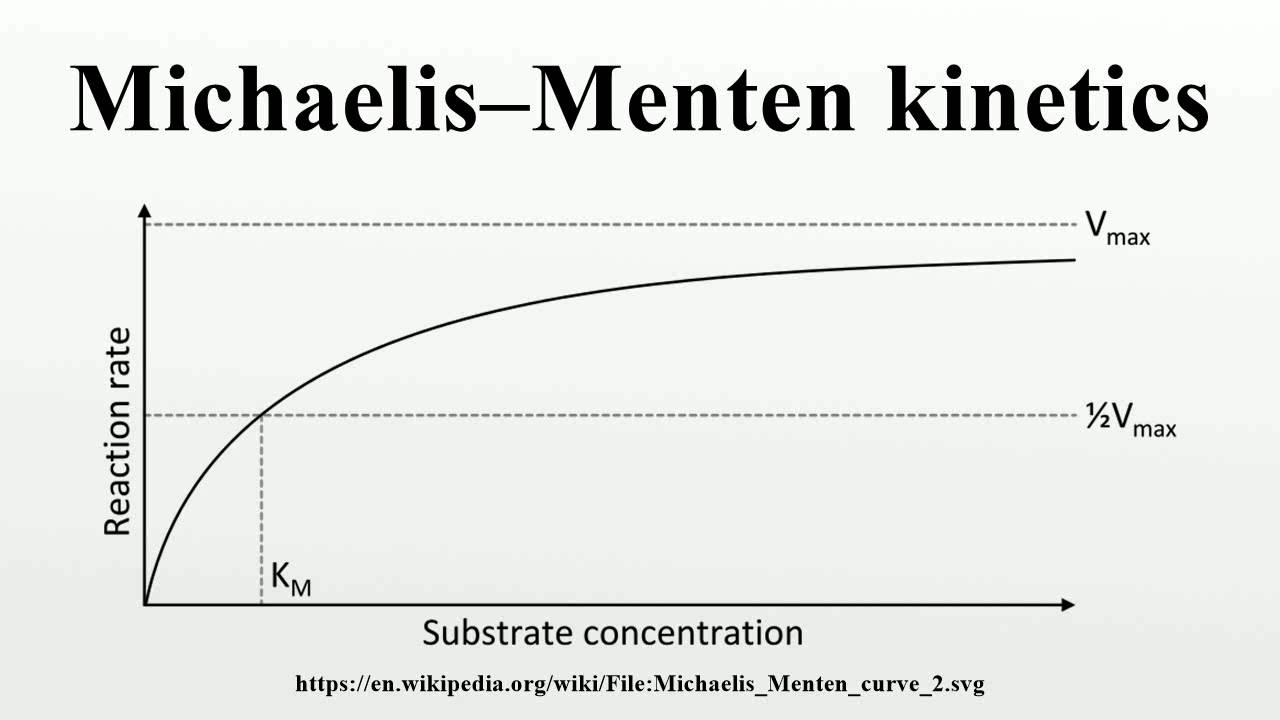
MichaelisMenten YouTube

ECUACION DE MICHAELIS MENTEN PDF
La cinétique des enzymes michaeliennes et l'équation de MichaelisMenten

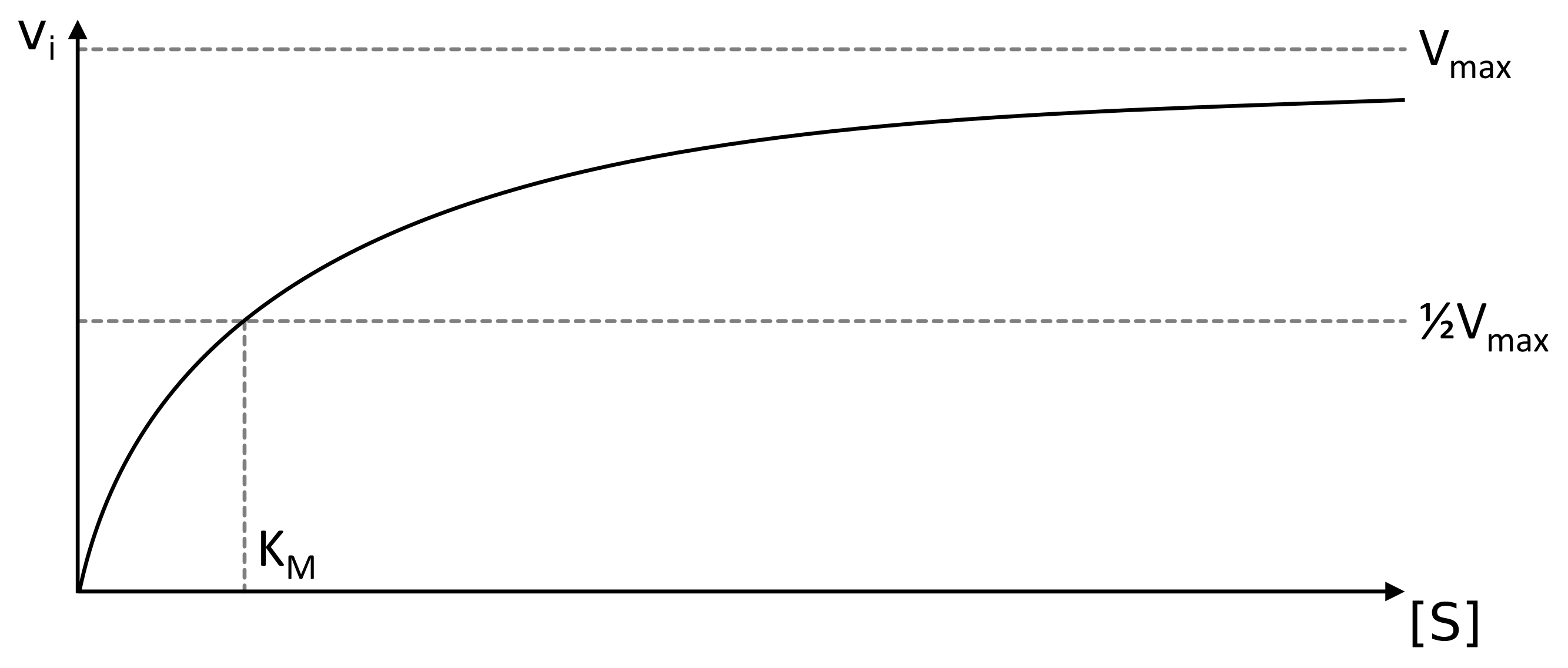
MichaelisMenten equation Interactive graph PhysiologyWeb

Michaelis Menten Equation Enzyme (PART 1) Introduction YouTube

044MichaelisMenten Equation YouTube

Developing a three‐dimensional animation for deeper molecular understanding of michaelismenten

PPT The MichaelisMenten Equation nicely describes the experimental observations. PowerPoint
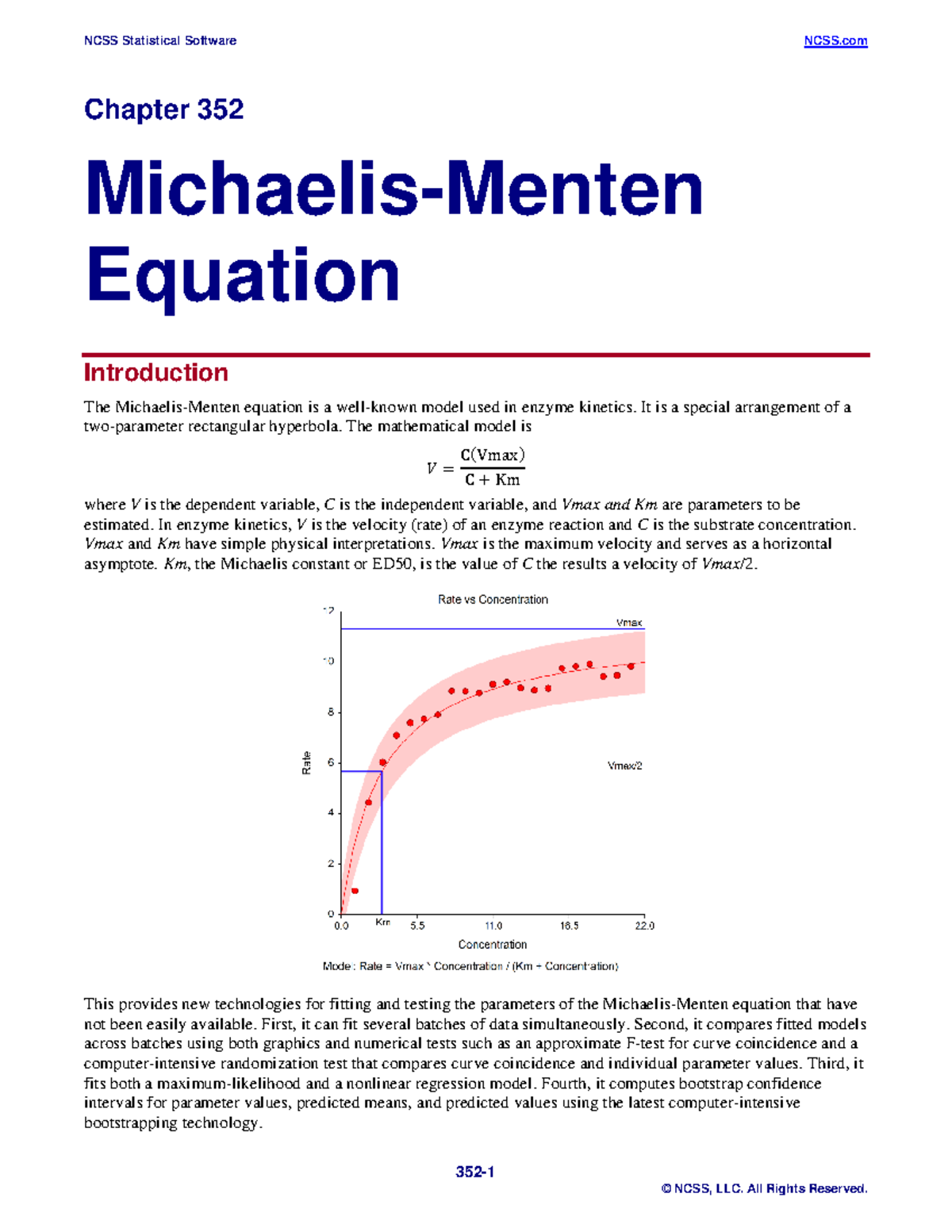
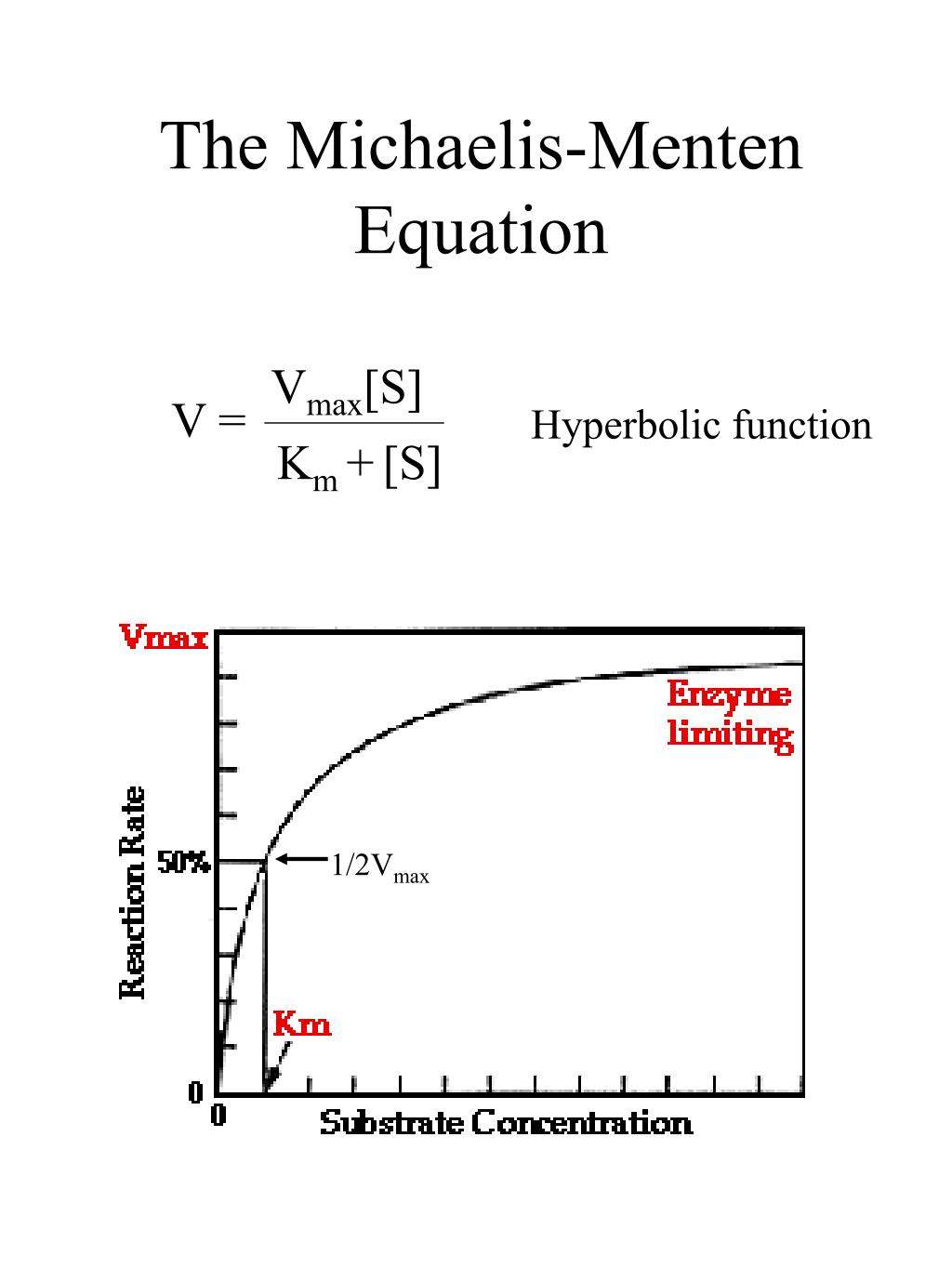
MichaelisMenten Equation It is a special arrangement of a twoparameter rectangular hyperbola

Deriving the Michaelis Menten Equation YouTube
PPT LAB 3 Enzyme PowerPoint Presentation, free download ID4526880
Significance of k m in Michaelis Menten equation. The K m is Michaelis constant and it can be defined as the substrate concentration at which the rate of reaction is half of the maximum velocity(V max).K m represents the affinity of an enzyme for a particular substrate. It means the lower the value of K m, the greater the enzyme's affinity for the substrate.. The Michaelis-Menten Equation describes the relationship between the rate of an enzyme-catalyzed reaction and the concentration of the substrate. It was named after the scientists Leonor Michaelis and Maud Menten, who developed it in 1913. If you've read about kinetics, these words may sound familiar. That's because enzyme-substrate.